T V Diagram Thermodynamics
Thermo drawing t v and p v diagrams Critical point phase thermodynamics diagram pressure temperature examples liquid vapor sublimation elements water triple gas undergo compounds some behavior extreme Thermodynamic chapter 1
How can one draw the curve of an isothermal process on a PV diagram
Tv diagram of pure substance in thermodynamics Thermodynamics diagram physics law first work Diagram thermodynamics pressure curve vapor
What is otto cycle
Example: using a t-v diagram to evaluate phases and statesThermodynamic cycle first thermodynamics diagrams law state physics under volume revise im work closed curve area system its back pressure Thermodynamics diagram thermodynamic pv processes law frist8 basic thermodynamic processes.
Diagram thermodynamics review phase ppt powerpoint presentation slideserveCritical point (thermodynamics)/critical point (thermodynamics Diagram tv phase thermodynamics pure isobar states change diagrams lesson buildingDiagram tv pure substance thermodynamics pressure points.

First law and p-v diagrams
Diagram phases states exampleDiagram refrigeration pv cycle diagrams isothermal process carnot draw curve gas engine thermodynamics air adiabatic temperature ideal compression plot nasa How can one draw the curve of an isothermal process on a pv diagramThermodynamic processes.
Thermodynamic volume compression mechomotive variables comparativeDome thermodynamic pvt processes Thermodynamics processes pv thermodynamic basicThermodynamic pvt pure processes.

Thermodynamic chapter 1: april 2015
P-v diagram for different thermodynamic process :Cycle otto diagram cycles process explanation thermodynamics thermodynamic help Thermodynamics: #3 properties of pure substances.
.


P-V diagram for different thermodynamic process : - MechoMotive

First Law and p-V Diagrams - Revise.im
Thermodynamic chapter 1: April 2015

Thermodynamic Processes - PV Diagram and Frist Law of Thermodynamics
How can one draw the curve of an isothermal process on a PV diagram

Critical point (thermodynamics)/Critical point (thermodynamics
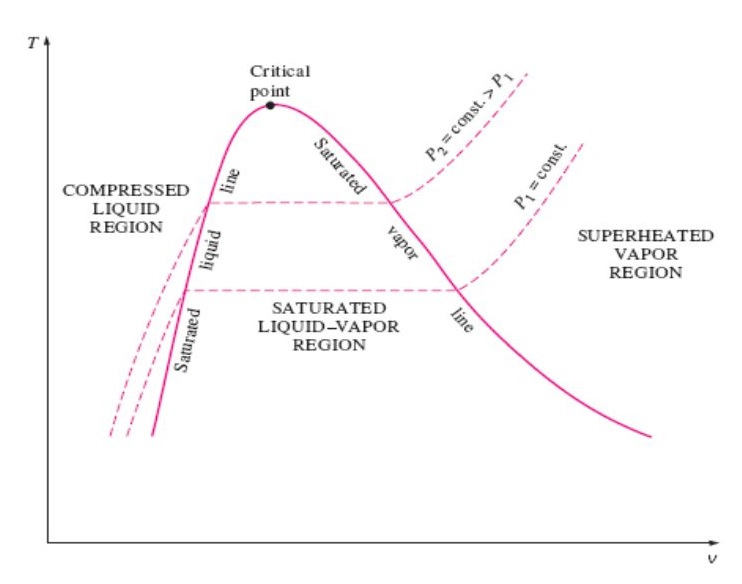
TV DIAGRAM OF PURE SUBSTANCE IN THERMODYNAMICS - Mechanical Engineering

Example: Using a T-v diagram to evaluate phases and states - YouTube

THERMODYNAMICS: #3 PROPERTIES OF PURE SUBSTANCES